It's a matter of affinity
TCR affinity is a quantitative measurement of the binding strength of the TCR to its target. So far, it has been shown that TCR affinity determines the fate and efficacy of T cells in immune responses. However, effective TCR affinity for cellular therapy is also dependent on the disease context and differs between autoimmune disease, infections or cancer. Therefore, our goal is to elucidate the mechanistic connection between optimal affinity and efficacy to improve T-cell therapy.
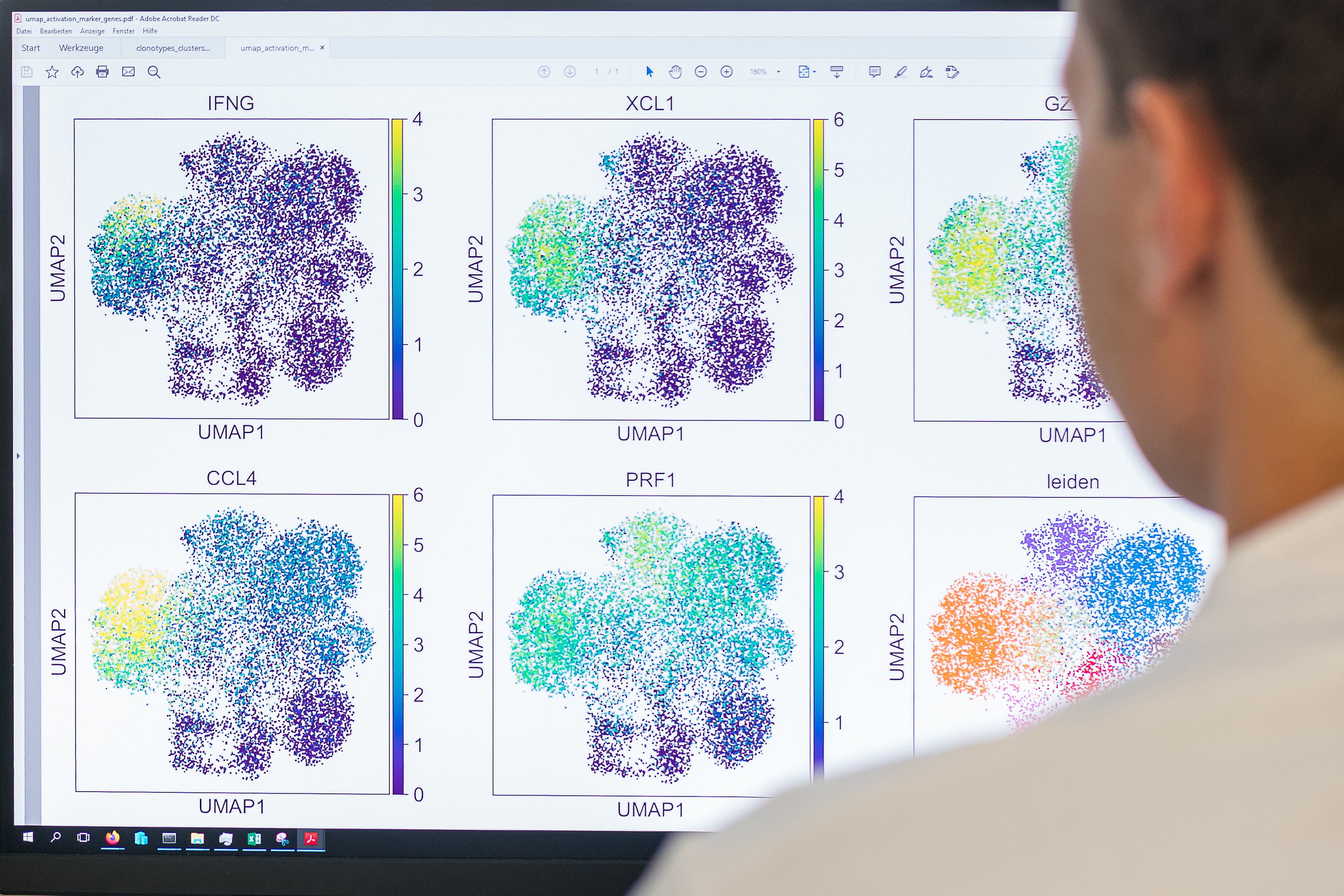
T cells undergo somatic recombination of the TCR during their development, which provides the possibility to generate an immense diversity of TCRs. It is estimated that a human individual can harbor up to 108 unique TCR sequences. Some of these generated TCRs recognize the same antigen and form antigen-specific precursor populations of naïve T cells. The size of antigen-specific precursor populations depends on the epitope and is estimated to harbor between 1 and 104 different clonotypes with a diverse avidity distribution. Antigen-specific adaptive immune responses arise from these polyclonal repertoires of naïve precursors. Upon antigen encounter, individual T-cell clones are recruited into the immune response, clonally expand and differentiate into memory and effector subsets. During this process, T cells with a high affinity TCR are preferentially selected during the acute phase of the immune response. However, depending on the disease context, epitope and type of immune response different TCR repertoires of different diversities are maintained over the course of clonal selection and time. TCR recruitment is governed by clear avidity thresholds, including a large number of low-avidity T-cell clones in primary immune responses. Although the immune response is quickly dominated by high-avidity T-cell clones, low-avidity T cells are still capable of forming small memory populations, providing a flexible niche for secondary immune responses. These memory populations can play a crucial role in protection against mutational escape variants, as commonly encountered in infectious disease. We seek to understand the role of low-avidity T cells and polyclonality in immune responses more clearly in order to improve vaccination strategies and cellular therapies.
Personnel


Related publications
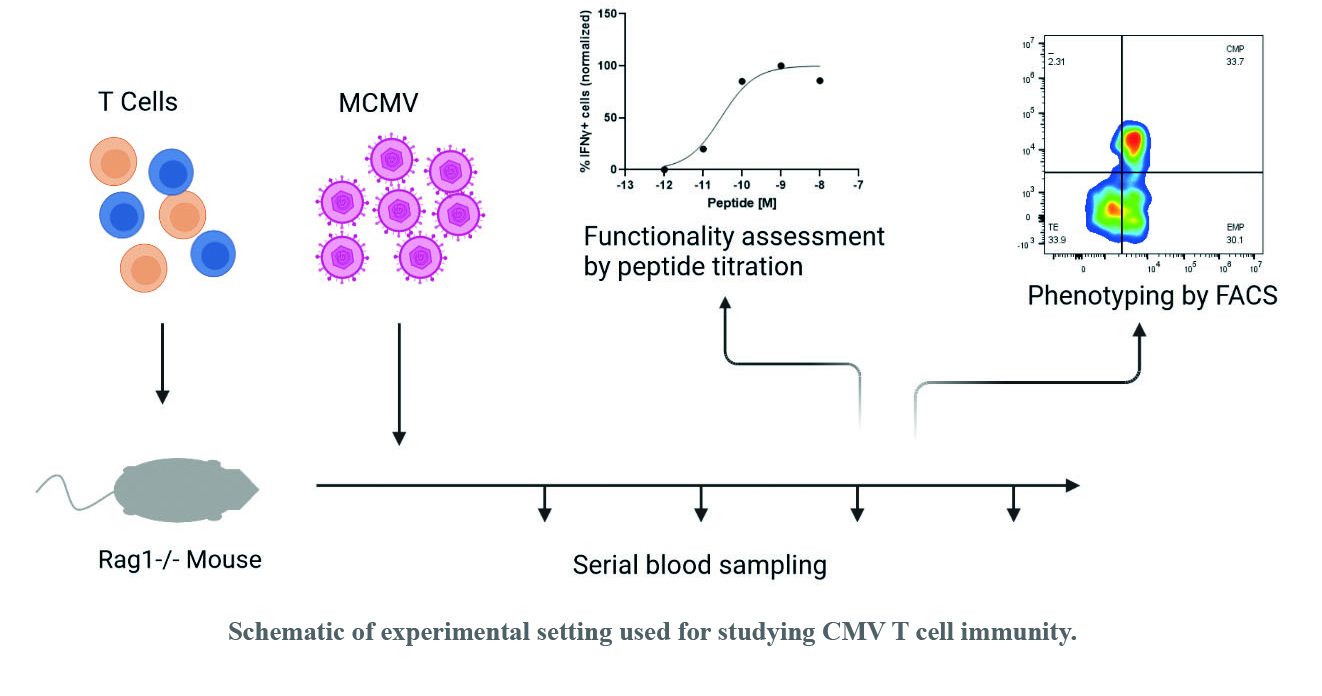
During acute infections, responding T cell populations expand to clear the pathogen, after which they retract to leave a small population of long-lasting memory cells. Infection of an organism by Cytomegalovirus (CMV), however, is never cleared, and can thus stimulate responding T cell populations for the lifetime of the organism. T cells repeatedly stimulated by CMV infection on chronic timescales develop into extremely large populations which do not contract, a phenomenon known as memory inflation. This chronic antigen stimulation does not exhaust the T cells however, which instead remain strongly differentiated yet functional.
Earlier work from our lab indicates that the composition of the inflationary population changes over time, with lower affinity TCR clones gradually becoming dominant relative to the higher affinity TCR clones which initially dominate the response. Despite this evolution of the population composition at the TCR clone level, the overall functionality of the population appears to be maintained. Because TCR affinity is associated with functionality, this implies there is a compensatory change of the functionality in the lower affinity TCR clones. We seek to investigate this with functional characterization of TCR clones within evolving TCR repertoires in the murine CMV model.
Personnel
Adoptive T-cell transfer of high-affinity chimeric antigen receptor (CAR)- T cells targeting CD19 has shown impressive clinical success in treating certain B-cell malignancies. However, broader clinical application has been hindered by the development of potentially life-threatening side effects like cytokine release syndrome, accompanied by neurotoxicity. Additionally, consistent strong activation results in CAR-T cell exhaustion, which could limit their long-term functionality and thus contribute to tumor relapses in treated patients. While modifications of the CAR molecule structure have shown some influence on efficacy and persistence, their impact on toxicity profiles of CAR-T cells has been limited studied. Recent evidence suggests that affinity could serve as a suitable parameter to balance safety and maintain CAR-T cell efficacy. However, a comprehensive understanding of how receptor binding affinities affect the functionality and safety of anti-CD19 CAR-T cells is still lacking. Gaining insights into the affinity threshold, persistence, and short- and long-term functionality of variable CAR molecules can potentially enhance anti-CD19 CAR-T cell therapy and enable the exploitation of its full potential in adoptive cell therapy.
Personnel



